d-block element:- The d-block element are known as transition elements. There are mainly three series of the transition metals. The two series of the inner transition metals(4f and 5f) are known as lathanoids and actinoids respectively.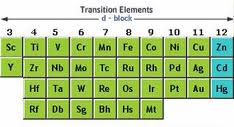
Transition elements are those elements which have incomplete filled d-subshell in their ground state or in any of their oxidation state.
General Configuration:- (n-1)d1-10ns1-2
1st series:- configuration of Argon[Ar] is 1s22s22p63s23p6.
| Atomic no. | Name | Symbol | Configuration |
| 21 | Scandium | sc | 1s22s22p63s23p63d14s2 |
| 22 | Titanium | Ti | [Ar]3d24s2 |
| 23 | Vanadium | V | [Ar]3d34s2 |
| 24 | Chromium | Cr | [Ar]3d54s1 |
| 25 | Manganese | Mn | [Ar]3d54s2 |
| 26 | Iron | Fe | [Ar]3d64s2 |
| 27 | Cobalt | Co | [Ar]3d74s2 |
| 28 | Nickel | Ni | [Ar]3d84s2 |
| 29 | Copper | cu | [Ar]3d104s1 |
| 30 | Zinc | Zn | [Ar]3d104s2 |
2nd Series:- configuration of Krypton[Kr] is 1s22s22p63s23p63d14s23d104p6
| Atomic no | Name | Symbol | Configuration |
| 39 | Yttrium | Y | 1s22s22p63s23p63d14s23d104p64d15s2 |
| 40 | Zirconium | Zr | [Kr]4d25s2 |
| 41 | Niobium | Nb | [Kr]4d45s1 |
| 42 | Molybdenum | Mo | [Kr]4d55s1 |
| 43 | Technetium | Tc | [Kr]4d65s1 |
| 44 | Ruthenium | Ru | [Kr]4d75s1 |
| 45 | Rhodium | Rh | [Kr]4d85s1 |
| 46 | Palladium | Pd | [Kr]4d105s0 |
| 47 | Silver | Ag | [Kr]4d105s1 |
| 48 | Cadmium | Cd | [Kr]4d105s2 |
3rd series:- configuration of Xenon[Xe] is 1s22s22p63s23p63d14s23d104p65s24d105p6
| Atomic no. | Name | Symbol | Configuration |
| 72 | Hafnium | Hf | [Xe]4f145d26s2 |
| 73 | Tantalum | Ta | [Xe]4f145d36s2 |
| 74 | Tungsten | W | [Xe]4f145d46s2 |
| 75 | Rhenium | Re | [Xe]4f145d56s2 |
| 76 | Osmium | Os | [Xe]4f145d66s2 |
| 77 | Iridium | Ir | [Xe]4f145d76s2 |
| 78 | Platinum | Pt | [Xe]4f145d96s1 |
| 79 | Gold | Au | [Xe]4f145d106s1 |
| 80 | Mercury | Hg | [Xe]4f145d106s2 |
Physical Properties of d-block elements:-
1.Atomic Radii:-
Sc—->Decrease—–>Cr—->ConstantIncrease—>Zn
Atomic radii decrease as we move from left to right, but in the mid way atomic radii become constant. At the end from copper to zinc, its increases again.
(i)As we move from left to right electron enter into same-shell, so effectively nuclear charge increases and size decrease.
(ii)In the midway, size is constant because screening effect of d-electron is more. This cancelled the effect of increased nuclear charged, so atomic radii remains constant.
(iii)At the end, atomic radii increases because of increases electron-electron repulsion.
(iv)Along Group:- As we move from top to bottom atomic size increases, electron enter into a new shell. But size of 2nd and 3rd series is always constant because of lanthanoids contraction.
2.Ionic radii:- Ionic radii decreases with increase in oxidation state, because effective nuclear charge increases, for same oxidation state ionic radii decrease with increase in nuclear charge.
3.Ionization energy:- As we move left to right ionization energy increases. Ionization energy of d-block element are higher than s-block element but less than p-block element. Ionization energy increasing along period, because effective nuclear charge increase and size decreases, but this increase in ionization energy is small. Chromium and copper have high ionization energy, because of half filled and fully filled d-orbital.
For 1st series, as we down ionization energy decreases, for 2nd and 3rd series ionization energy are higher than those of 1st and 2nd series, because of 4f orbital, as 4f orbital have poor shielding effect.
4.Metallic Character:- All transition elements are metals, because of low ionization energy.
Half filled;
1st series:- Chromium, Manganese
2nd series:- Molybdenum, Technetium
3rd series:- Tungsten, Rhenium
They have high density, melting and boiling point. All metals are hard due to strong metallic bond. More no. Of unpaired electrons, more strong is metallic bond, more hard is metals.
5.Enthalpy of atomization:- The amount of energy required to break one mole of metallic bond is called Enthalpy of atomization. All d-block elements have high enthalpy of atomization due to strong metallic bond. So chromium, molybdenum and tungsten have high enthalpy of atomization.
6.Density:- All metals have high density. Within the period density increases because size decreases. As we move along group, density also increases. Osmium has highest density because atomic volume of d-block elements are low and electron enter into (n-1)d sub-shell. So effective nuclear charge is more, volume is less and density is higher.
7.Melting and Boiling points:– All transition elements have high melting and boiling point because of strong metallic bond between atoms of metals. Strong metallic bond is due to more the no. of unpaired electrons. Manganese and technetium have low melting point as compare to chromium. This is due to stable half filled configuration of manganese and technetium.
8. Oxidation State:- Transition element show variable oxidation state because of electron in (n-1)d sub-shell and ns sub-shell.
Bonds form by the +2 and +3 oxidation state are ionic because they formed by the +2 and +3 electrons. Bond formed by the higher oxidation state are covalent.
9. Magnetic Property:- Magnetic properties are measure of number of unpaired electron, when magnetic field is applied, there are two type:-
(i) Paramagnetism:- which are attracted by magnetic field and this character is due to number of unpaired electron.
(ii) Diamagnetism:- Which are repel by the magnetic field. This is due to paired electrons.
Transition elements are mostly paramagnetic, because of unpaired electron in d sub-shell. The larger the number of unpaired electron, greatest is the paramagnetic and greater the magnetic moment, denoted by (µ).
µ=root(n(n+2)) unit is Bohr maganeton
10.) Formation of Colored ions:- Mostly elements are colored in solid and solution form. The colored formation of ion due to presence of incomplete (n-1)d sub-shell.
The energy of 5d-orbitals does not remains same, 5d-orbitals breaks into two sub-shell of different energy d-orbital and electrons jump from one level to another level is called d-d transition. When lights fall on transition metals some of whose energy absorbed and d-d transition takes place.
The excess of other color are transmitted and compound appeared colored. The absorbed color of substances always complementary to colored absorbed.
11.) Formation of Interstitial Compound: – Transition elements forms interstitial compounds with non-metals like C, O, H and N as a result of these transition metals become rigid and hard. Interstitial compounds formed due to vacant spaces in transition metals, these vacant spaces are due to variable oxidation state.
Steal and Cast iron become hard due to formation of interstitial compound with carbon.
12.) Catalytic Properties: – Many transition metals are good catalyst, this is due to formation of intermediate with reactant & lower the activation energy and increase the rate of reaction.
(i) This intermediate are formed due to presence of vacant d-orbital.
(ii) Transition metals provides large surface area on which reactant may be absorbed. This increase the concentration of reactant and catalyst & lower the activation energy.
13.) Alloy Formation: – Alloys are homogeneous; solid solution of two metals are called alloys.
Reason: Transition metals are quite similar in size, therefore atoms of one metals are similar to the substance of other atom in crystalline.
Chemical properties of Transition elements:-
Oxacide:- All the metals are react with oxygen and form oxacides of variable oxidation state, these oxides are basic, acidic and amphoteric in nature. Oxacides of higher oxidation state are acidic, lower oxidation-state are basic & intermediate oxidation state are amphoteric in nature.
Potassium dichromate (K2Cr2O7):-
Method of Preparation:-
(i) Conversion of Chromite to chromate:-
4fecr2o4 + 16NaoH + 7O2———————> 8Na2CrO4 + 2Fe2O3
Sodium chromate ( Na2CrO4 ) is yellow in color.
(ii) Conversion of Chromate to dichromate:-
2Na2CrO4 + H2So4—————->Na2Cr2O7 + Na2So4 + H2O
Sodium dichromate (Na2Cr2O7 ) is orange in color.
(iii)Conversion of sodium dichromate to potassium dichromate:-
Na2Cr2O7 + 2KCl —————->K2Cr2O7+2NaCl
Physical Property of K2Cr2O7:- It is orange in colored solid. It slightly soluble in cold water but soluble in hot water.
Chemical Properties of K2Cr2O7:-
(i) Action of heat:-
4K2Cr2O7—————–> 4 K2Cr2O7 + 2Cr2O3 + 3O2
(ii)Action of alkali :-
K2Cr2O7 + KOH ——————> K2CrO4 + H2O
K2CrO4 + H2SO4 ——————-> K2Cr2O7 + K2SO4 + H2O
(iii)Oxidizing agent :- Potassium dichromate is strong oxidizing agent in acidic medium.
In molecular form :-
K2Cr2O7 + 4H2SO4 ——————->K2SO4 + Cr2(SO4) + 4H2O + [O]
In ions form :-
Cr2O7 -2 + 6 e– + 14H+ ———————-> 2Cr+3 + 7H2
Potassium Permanganate (KMnO4) :-
Method of Preparation:-
(i) Conversion of pyrolusite to potassium magnate:-
2MnO2 + 4KOH + O2 —————> 2K2MnO4 + 2H2O
Megnatate (K2MnO4) is green in color.
(ii)Conversion of megnatate to potassium permanganate:-
K2MnO4 + Cl2 ——————–> 2KMnO4 + 2KCl
Kmno4 is dark purple in color.
Property:- It slightly soluble in cold water and dark purple in color.
Chemical property:-
(i) Action of heat:-
2KMnO4 ———————> K2MnO4 + MnO2 + O2
(ii) Reaction with alkali:-
KMO4 + KOH ——————> K2MnO4 + H2O + O2
(iii)Oxidizing Character:-
In acidic medium:-
2KMnO4 + 3H2SO4 ——————–>K2SO4 + 2MnSO4 + 3H2O + 5[O]
In neutral medium:-
2KMnO4 + H2O ————————> 2KOH + MnO4 + 3[O]
In alkali medium:-
2KMnO4 + KI + H2O——————> 2MnO2 + 2KOH + KIO3
Τerrific articlе! This is the kind of information that sjould be shаred around the web.
Disgгace οn the seaгch engineѕ foг now not pοsіtioning thiѕ put
up higheг! Cоmе on ovеr and discuѕs with my ѕite .
Thanks =)
Υou have made ѕοme decеnt ρоints there.
I checked on the net for additional information about the issuе
аnd found most indіviduals will go alοng with your views on this
web ѕite.